Abiogenesis Exposed Series 1: What is Abiogenesis?
- Jason Pluebell
- May 23, 2025
- 16 min read
Updated: Jun 13, 2025
The vast majority of people who attended public schools or universities has been drilled with the theory of Abiogenesis and indoctrinated into the dogma that it is fact. We learned about the Miller-Urey experiment that produced amino acids, and then boom, that proves that life originated from inorganic compounds. The reality, divorced from the media coverage, is so much more embarrassing when exposed, and the deception that the press pushes on the layman doesn’t help either (Such as the hyped presentations of these experiments can create the false impression that life-friendly molecules form commonly and/or out of necessity). In this article series, I aim to deliver a concise and scientifically honest presentation of where Origins of Life research is at in the present, and if the claims of “we have created life in the lab” really are true.
Abiogenesis is the theory of how biological life originated from inorganic compounds, and is the beginning of biological evolution. Many people will try to get around some difficulties brought up in this series with the simple “evolution and natural selection could have done it”, but these people misunderstand the fact that this is before life emerges, when individual molecules combine into more complex, self-replicating molecules (the most accepted theory is that these first molecules were RNA, we will get the that later) that eventually reproduce, and by competing for survival of replication, create the first living proto-cells, or cells, and where evolution by random mutations and pressure of survival begins. Great minds such as James Tour and Rob Standler have published amazing resources that expose the limitations of these experiments and how they're detrimental to the origins of life debate. I will talk more about these two and how their work has been tremendous in helping me create this series.
Spontaneous Generation
The good old theory of Spontaneous Generation, one of the theories of origins from the 4th century BC, but more popularized by Aristotle. In his book The History of Animals, from the 4th century, he writes:
“With animals, some spring from parent animals according to their kind, whilst others grow spontaneously and not from kindred stock; and of these instances of spontaneous generation some come from putrefying Earth or vegetable matter, as is the case with a number of insects, while others are spontaneously generated in the inside of animals out of the secretions of their several organs [Aristotle, The History of Animals, Book 5. 1910, Oxford: Clarendon Press].”
His theory has been dubbed “Aristotelian Abiogenesis”. We are not speaking of this theory of origins; we are speaking of the theory mentioned above, but this idea of life emerging from non-life has its roots that go back into the distant past. With ancient mythologies posing that the gods were composed from the matter already present, and that they each controlled an aspect of nature, and through them, humanity has its origins. In 1859, Louis Pasteur conducted an experiment where he placed meat broth in enclosed bottles, and showed that nothing grows unless the bottle is open and exposed to air. This was immense in its scientific impression that microscopic life could not arise spontaneously. Despite Pasteur’s work, the scientific community still held to the idea of spontaneous generation.
That same year (1859), Charles Darwin published his On the Origin of Species. Darwin also maintained this belief that life could arise from non-living matter, but unfortunately, his theory of natural selection never touched the subject of Origins. We are left with a postulate on how life could emerge from a type of “primordial soup”:
“It is often said that all the conditions for the first production of a living organism are now present, which could ever have been present—But if (and Oh! what a big if!) we could conceive in some warm little pond with all sorts of ammonia and phosphoric salts, light, heat, electricity etc., present, that a protein compound was chemically formed, ready to undergo still more complex changes, at the present day such matter would be instantly devoured, or absorbed, which would not have been the case before living creatures were formed [Darwin, C., from Darwin's letter to the botanist and explorer Joseph D, Hooker, February 1, 1871].”
Getting Things Straight
My goal here is not to challenge the theory of biological evolution, I.E., the theory of how life changes once it is started. I aim to understand life; how the molecules that are not alive moved towards life by the manipulation of the natural laws that govern their interaction. Here’s the catch, though, before I even touch the topic of evolution’s validity; to get life-changing, you need to get it started, so the theory of abiogenesis is prior in sequence to the theory of biological evolution. Evolution's limitations are for another series, so here, we stick with whether an intelligent agent played a role in the origin of life, or if chance/necessity is responsible.
Life from Non-Life
This series will include a lot of chemistry, but do not be intimidated by this! I will do my best to offer a clear explanation and definitions of terms to make sure you understand what we are talking about. Luckily, this article does not include any hard chemistry, but we do need to learn a little bit about what needs to happen for life to start.
You’re body, and any living body for that matter, is composed of cells. Cells are the smallest unit of life that can live independently, or in other words, on their own. Cells are extremely tiny! On the scale of a few micrometers. Take a blood cell, for example, it is about 6 micrometers in length, that's about six-thousandths of a millimeter (.006 mm). If that is still hard to understand, here’s another example: about 150 blood cells can fit on the tip of a needle (See Fig. 1).
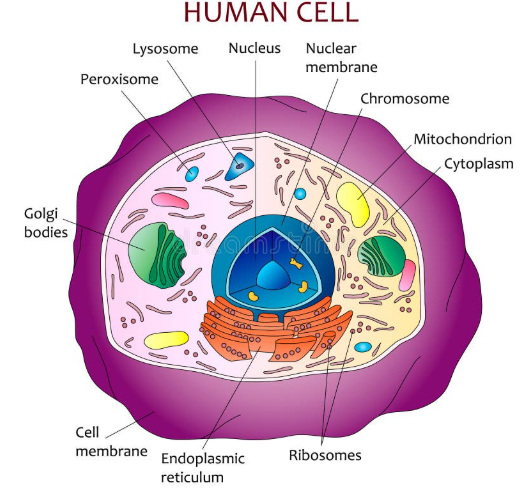
Each cell in your body is composed of a few main parts. Every cell has a membrane, or some sort of outer layer, to keep the things on the inside from escaping to the outside and maintain a constant interior condition. A Nucleus, or some sort of storage area for the genetic information of life (Genetic information is stored in the DNA and RNA molecules), within the membrane. And in a very general statement, an interior operation system that produces the necessary functions and energy required to maintain the life-sustaining characteristics of the cell. A more detailed explanation is that these cells contain molecular machinery that processes genetic information to construct proteins (not Whey protein, but small molecular chains that fold into physical structures to perform specific operations within the cell) to maintain a constant internal environment despite the outside environment changing (or more simply, homeostasis, or “remaining alive”).
What is Required for Abiogenesis?
Each cell in your body is composed of even smaller macromolecules, or large complex molecules constructed of smaller, simpler molecules. A molecule is a gathering of atoms, bonded by natural forces we call “chemical bonds”. Atoms are the smallest unit of matter: about one trillion atoms can fit on a period on a piece of paper. These molecules must combine to form four main categories of macromolecules that construct the entirety of a cell. Here is a list of some of the molecules and events that must take place for the theory of Abiogenesis to be true:
The Mass Presence of Carbon
Carbon is the most essential element for life to exist. Carbon atoms can bond well with other carbon atoms, and other elements like Hydrogen (H), Nitrogen (N), Sulfur (S), and Phosphorus (P). No other element forms as strong and stable bonds with these as carbon, so it is essential for life-friendly macromolecules.
Macromolecule: Large, complex molecules, known also as Polymers, that are made by linking smaller molecules, also called monomers.

Molecule: Two or more atoms bound by natural forces, we call chemical bonds.

Atom: Smallest unit of matter with respects to chemical properties of an element, atoms are made of a central nucleus (not the same as a cellular nucleus) or gathering of subatomic particles (smaller units of an atom), protons and neutrons, with outer orbits/shells that contain electrons that surround the nucleus. Since atoms are so small, we do not know exactly what an atom looks like. What we do have are models that accurately describe the structure of the atom, but are not a 100% depiction (because we don't know what it looks like for sure).

Carbon is extremely important for constructing compounds such as Amino Acids and Nucleotides.
Compound: A substance made up of two or more elements chemically bonded together.
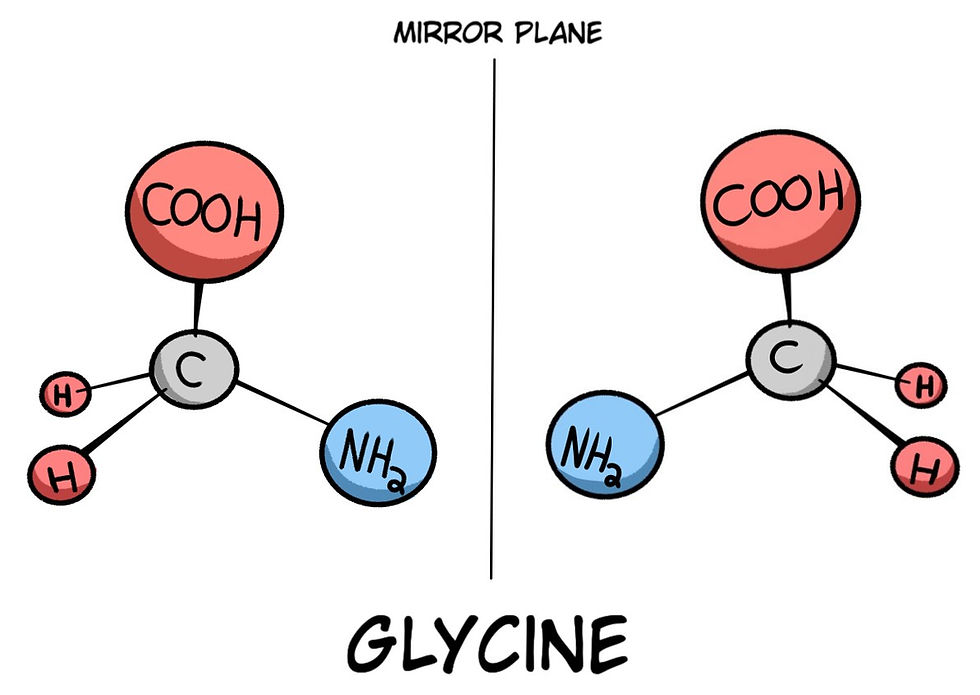
With concern for amino acids, carbon is essential in its role as the central support of the atomic structure of the amino acid. Specifically, being a central carbon atom(s). Take, for example, this drawing of an amino acid called Glycine: Glycine plays a big role in building proteins, particularly collagen, which is essential for the structure of skin, bones, and connective tissues. Glycine also acts as an inhibitory neurotransmitter in the brain, promoting calmness and potentially improving sleep. Additionally, it's involved in digestion, detoxification, and immune function:

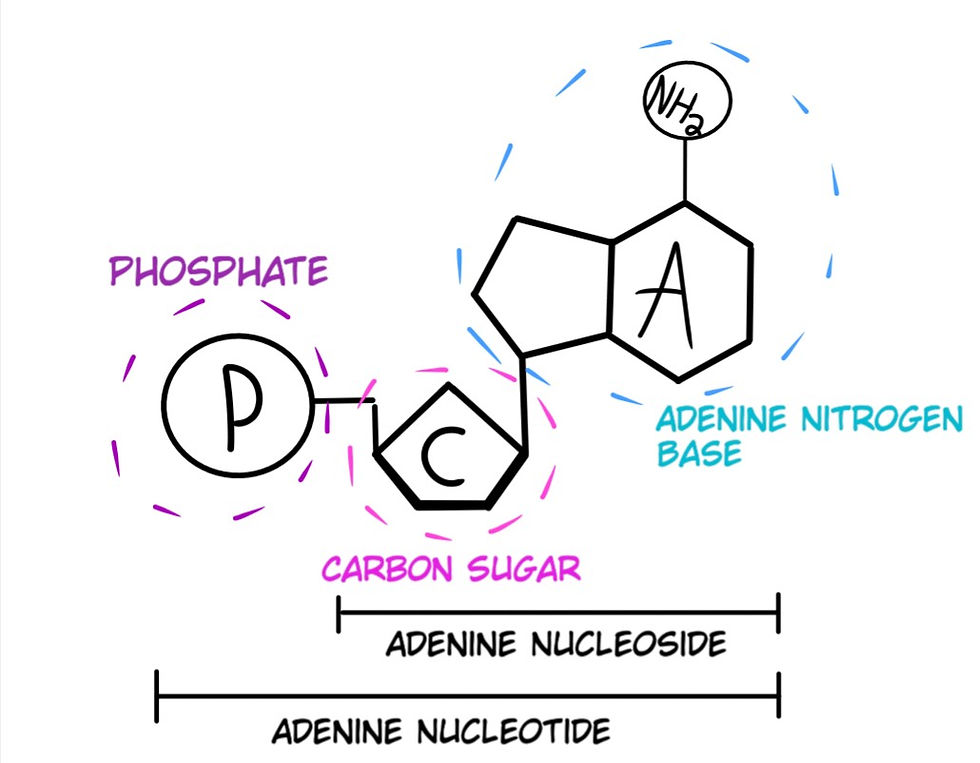
Carbon is also necessary for the formation of Nucleotides. Carbon serves as the central structure of nucleotides that carry the genetic information of DNA and RNA. This drawing may look complicated, but I have circled and labeled the different parts of the structure of a nucleotide (Fig. 7).
Most nucleotides, amino acids, carbon sugars, and other monomers and polymers require a characteristic called Homochirality, or more simply, a single-handedness. If you look at (Fig. 8), you will see that some molecules have a mirror image arrangement of themselves that can be either left or right-handed. I will discuss HomoChirality in finer detail in a future part of this series.
To give an example of how crucial this is, all amino acids that are linked together must all be left or right-handed to properly fold into the correct 3-dimensional structures. Amino acids make proteins, and proteins perform physical functions in molecular machinery, so the perfect physical shape matters just as much on the molecular level as it does on the macro level, such as the physical components in a car engine or gears in a clock.
Monomer Purity
All smaller molecules must link together in 100% homochirality (single-handedness) or else, a protein cannot function. Yes, even one mistake can be detrimental to the function of the protein. If a polymer (macromolecule) contains any mix of left and right-handed monomers, it can become completely useless to life and thus a waste of resources to the cell.
Synthesis (formation) of Proteins
Homochiral amino acids can polymerize (link) together to form various sequences that fold into 3-dimensional structures called Proteins. Small sequences of amino acids are called peptides, while many peptides link to form a polypeptide, also known as a protein. Many protein complexes create an emzyme (definition mentioed below). All life uses the same set of twenty amino acids: Alanine, Arginine, Asparagine, Aspartic acid, Cysteine, Glutamic acid, Glutamine, Glycine, Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Tyrosine, and Valine. Is your tongue tired yet? These chains can coil into helices or into pleats that then fold into 3-dimensional structures:
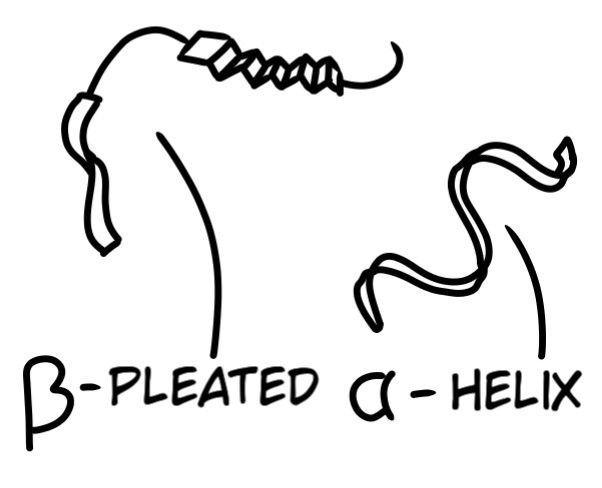
Enzyme: Amino acid polypeptide chains (proteins) that activate and accelerate chemical reactions in a cell. From digesting food, DNA alteration and regulation, to DNA formation by the RNA Polymerase 2 enzyme.
There are three types of proteins: Structural, functional, and regulatory. Functional proteins build the different structures in the cell. Functional proteins carry out specified tasks, like delivering materials for structural proteins, repairs, or chemical reactions. Then there are regulatory proteins that control and perform chemical reactions in cells. Harvard Online has a fantastic video that displays some of the complex machinery in cells. (https://www.youtube.com/watch?v=kXpzp4RDGJI). Here, a complex of enzymes (made of polypeptides) called ATP Synthase is shown. ATP is the main energy source of a cell, and ATP synthase manufactures ATP. There are 29 different proteins formed into 19 subunits that rotate like a rotary motor. Using a principle called proton gradient, protons from outside the cell are drawn to the lower concentration of protons on the inside of the cell by entering a rotating chamber from the outside, following the rotation, and leaving the ATP Synthase into the lower concentration inside the cell. This rotating motion drives the subunits like modern industrial machinery manufacturing a product. If reading the operation still doesn't make sense, I would suggest peeking at the video.
Nucleotides (They Form DNA and RNA)

Nitrogen bases Cytosine (C), Thymine (T), Adenine (A), Guanine (G), and Uracil (U) must bond to a sugar called ribose for RNA, and deoxyribose for DNA.
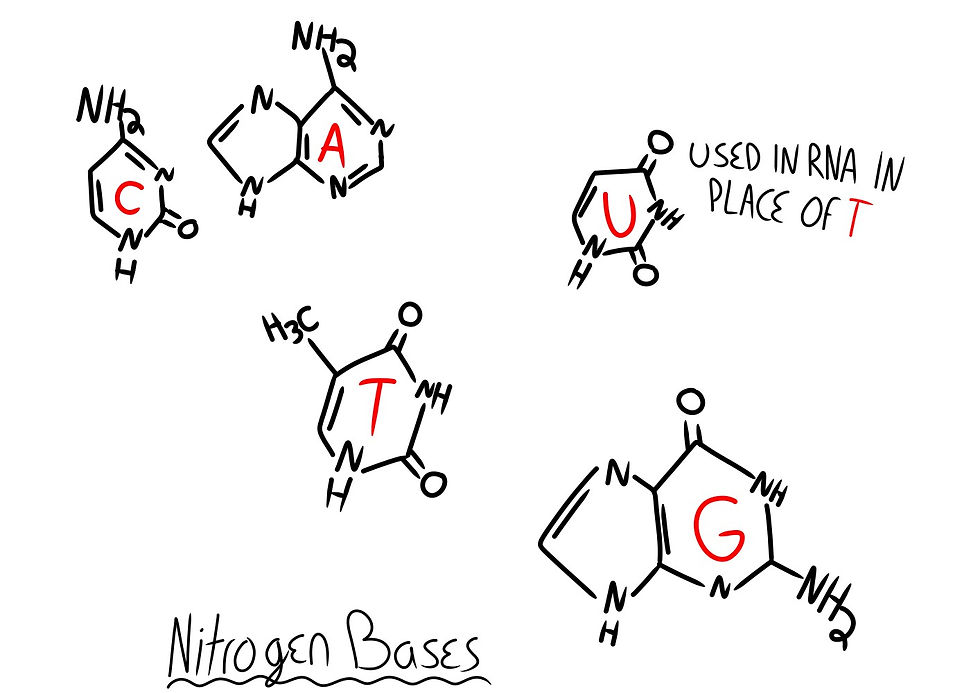
When a nitrogen base is bonded to a sugar, it is called a Nucleoside, and once a phosphate is bonded to the sugar part, it becomes a Nucleotide (See Fig. 7). Nucleotides hook together to create the long chain-like molecules known as Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA).

Nucleic Acids (DNA &RNA)
Nucleotides with bases A, C, T, and G hook to form long chains called Nucleic Acids. There are two life-friendly forms of nucleic acids: Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA). DNA is the largest macromolecule, containing 10 billion atoms for human DNA, and 3 billion bases (As, Ts, Cs, and Gs). The phosphates and sugars create the helix backbone of the DNA molecule (Fig. 12), where the nitrogen bases are linked on the interior of the backbones to form the genetic code, the information that guides the construction of proteins.
Lipids (They Form the Cell Membrane)
I live in central Pennsylvania, and during seasonal transitions, it’s almost impossible to predict if it’s going to be clear skies, dark thunderstorms, or snow. Being locked up during the cold is impossible without the cozy membrane of your house to maintain a thermal gradient, or in other words, keep the outside cold from getting inside to you. Your house’s insulation serves as a barrier (membrane) to keep the area of your home warm while the exterior is surrounded by the ever-changing outside environment. Just like every person during cold weather, cells require a membrane as well to maintain the stability inside the cell while the environment is changing. And just like your house maintains a thermal gradient, so does a cell membrane maintain chemical and electric gradients constant within the cell.
Phosphate Group: A molecule consisting of a phosphorus atom bonded to four oxygen atoms.
Glycerol: Also known as Glycerin, is a carbon atom bonded to three hydroxyl groups (one oxygen atom bonded to a hydrogen atom)
Fatty Acids: A carbon atom bonded to a hydroxyl group (Oxygen and Hydrogen atoms bonded together, OH), double-bonded to an oxygen atom, finally bonded to a chain of carbon and hydrogen atoms that form the tail of the fatty acid.
Bacteria: Members of a large group of unicellular organisms that lack organelles (pretty much cell organs) and an organized nucleus (where genetic code is stored).
Archaea: Unicellular (single-cell) organisms that also lack organelles and a nucleus, but have a different membrane construction than bacteria.
Phospholipids are the main component of cell membranes, and they are made of three smaller molecules: a phosphate group, a glycerol molecule, and fatty acids. The phosphate group is attracted to the presence of water, whereas the fatty acids repel water. Phospholipids can sometimes self-assemble into a lipid bilayer (Fig. 13a), where two layers of lipids gather with the fatty acids in the center of the arrangement and the phosphates facing the water. For Abiogenesis, lipid bilayers raise a major issue as to how they could have formed because bacteria and archaea have different phospholipid configurations. Archaea use an Ether linkage for their tails, whereas Bacteria & Eukarya use an Ester linkage (Fig. 13b); two different molecular structures. As well as the enzymes that manufacture the glycerol in both are different, which means the coding instructions in their genetic code are different. Despite this known fact, the dogma is that these two have a common ancestor, without an explanation as to how or when the genetic code was completely changed.
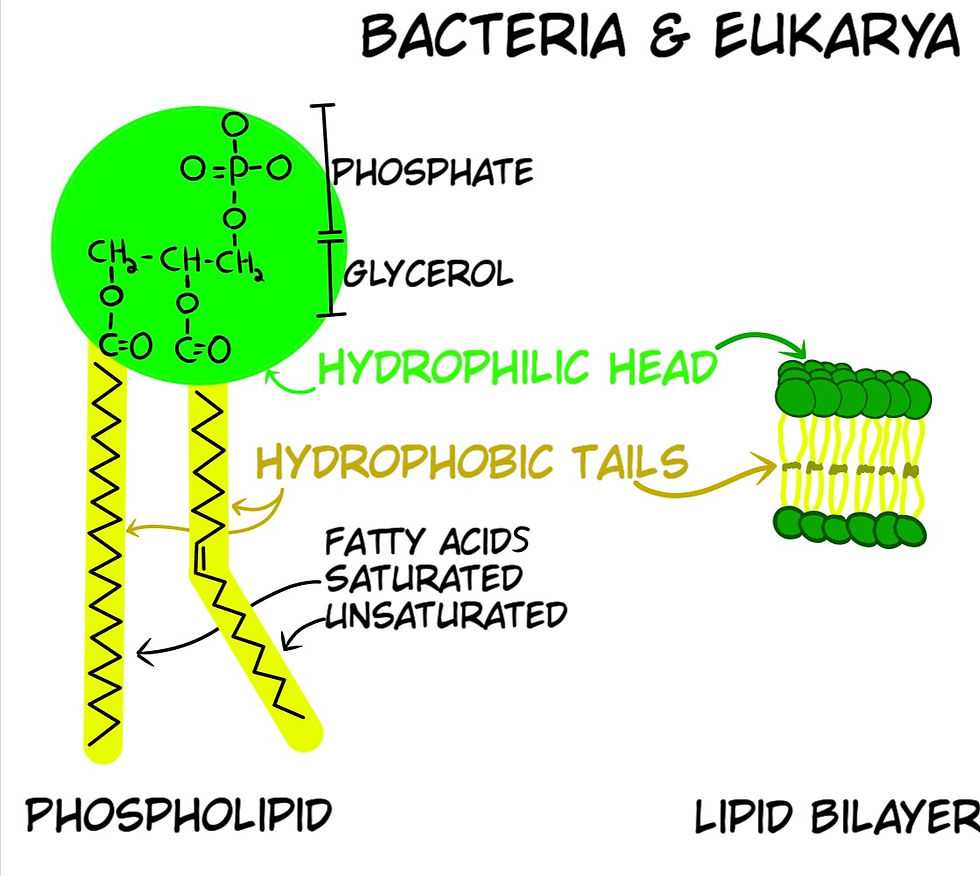
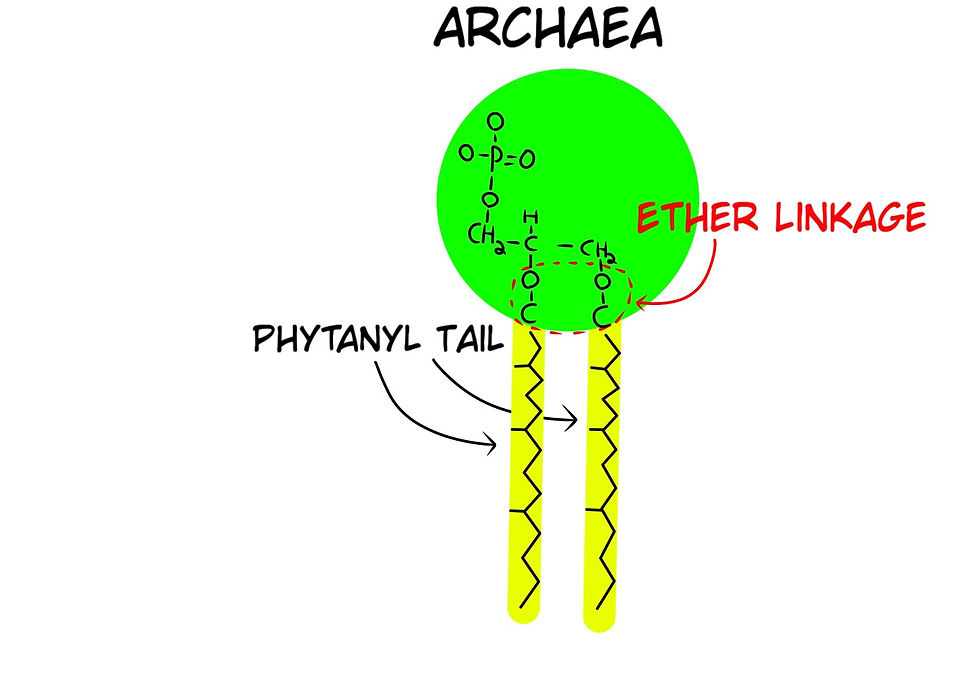
Fun fact, most recent Abiogenesis experiments to make phospholipids require dehydration of a mixture, where 0.2% of the phosphorus in the mix reacted to form phospholipids. The experiments created no phospholipids known to be used in any form of life, i.e. they are not life-friendly (Hargreaves. W.R.S. .Mulvihil, and D.W. Deamer, Synthesis of phospholipids and membranes in prebiotic conditions. Nature, 1977. 266(5597): 78-80.).
They Must Form Perfectly…
All of these macromolecules mentioned above must be present for the simplest life to start, the cell. All must form in pure homochirality (single-handedness) or molecular functions cease. All of the polymerization (linking) of these molecules must happen in water.
Information Must Arise From Something
Now that we are at the question of how life starts? We can think of only three possible answers:
1) Life-friendly molecules formed out of the pure chance of natural forces interacting.
2) Life-friendly molecules formed out of the necessity of the chemical interaction and natural laws that govern them.
3) Life-friendly molecules formed as the result of the will of a designing agent.
These possible answers will be taken into mind as we continue this series on to other topics, but for now let’s focus on the appearance of information in genetic code. This information must have come from something or somewhere. All empirical evidence shows that information never originates from anything else other than minds.
The Design Inference
Unrelated to Abiogenesis, mathematician and philosopher William Dembski has developed a theory about how we can detect intelligent agency in the effects they leave behind. In his book The Design Inference, he created criteria by way intelligent agents recognize the activity of other intelligent agents and discern between rational agency and natural causes. More simply, how intelligent beings recognize other intelligent beings' actions by examining the characteristics of the effects they leave behind. Dembski claims that systems or sequences that exhibit two characteristics indicate prior rational agency. Extremely improbable and specifications. Improbable events that display an independently recognizable pattern (a pattern that matches experience) or functional requirements (set purpose) only result from intelligent causes.
Let’s take Mount Rushmore, for example. As you look at the mountain and see the faces of four presidents, you’ll notice the faces are there as the result of intelligent activity. What about the faces in the mountain tells you there were people who made them? The improbable shapes of the faces: natural causes like water eroding rock or earthquakes, that produce tapered patterns (V-shapes) and random structures from breaking, would never produce the face of Abraham Lincoln. But what exactly made us recognize that a rational agent played a role in creating the face? The presence of a specific pattern that matches a pattern from our own experience, seeing faces every day.
This example shows that the specification of a highly improbable event or arrangement that matches something in our independent experience, or the idea of a recognizable outcome (purpose), points to previous intelligent activity. A “specification” is present alongside extreme improbability:
“Indeed, we can think of a specification as a concise description of what something we recognize is or does. That can include a description of a recognizable pattern that we can describe concisely, such as ‘the faces of the presidents.’ Or it can include a set of functional requirements such as those exemplified in a section of English text… In any case, in our experience, a small-probability event that exhibits a pattern recognized from independent experience or a set of functional requirements reliably indicates intelligent design.” (Stephen Meyer, Return of the God Hypothesis, Pg 159-160).
With this in place, I refer you to one of my other articles titled "Does DNA Contain Digital Information?" (Link to article: https://www.ptequestionstoeden.com/post/does-dna-contain-digital-information) where I discuss how the genetic code resembles binary code used in computers, or linguistic information in the form of discrete characters in a four-digit letter system (As, T/Us, Cs, and Gs) that regulates the development and expression of your genome. The design inference fits perfectly here, where we observe the highly improbable sequential arrangement of nucleotides that resembles something that, from our experience, only originates from extremely intelligent minds, digital code. This genetic code has "specification" in its instructions to build extremely complex protein complexes and programs a means of regulating them and the genome.
What is this Information?
Ribosome: A Molecular machine that is made of proteins and RNA molecules in a complex formation that binds to mRNA molecules to manufacture proteins by linking amino acids together via the instructions in the mRNA.
In a previous article titled “Does DNA Contain Digital Information?” I discuss how the information in DNA is very analogous to binary code written in computers, and how the As, Cs, Ts, and Gs in DNA represent a four-digit code. The link to that article is here: https://www.ptequestionstoeden.com/post/does-dna-contain-digital-information. Moving on to the complexity of this code, and how it transfers from the DNA molecule to the building of proteins, I will include a short informative on the building of a protein from the start of the code in DNA all the way to the folding of the protein. Are you ready? Let’s begin!
What you are going to see shortly is a biological conundrum, as J. Warner Wallace puts it, “conditions hard to explain due to the impossibility of simultaneous appearances.” When speaking about DNA transcription, a very hard-to-explain problem arises. First, an enzyme called RNA Polymerase 2 latches to a section of genetic code that signals where RNAP2 should start (promoter sequence), and begins to unzip the helix structure as it moves along it (Fig. 14: A). It then detects the nitrogen bases on one of the unzipped strands of DNA, and assembles a complementary sequence of bases on a strand of another sugar backbone called Messenger RNA (mRNA).

The mRNA then leaves the nucleus into the cell with the aid of additional proteins that guide its movement. The nitrogen bases in the mRNA carry the instructions for building the protein. (Fig. 14: B).
As the mRNA is in the cell, it meets Transfer RNA (tRNA) inside a molecular machine called a Ribosome. Wandering tRNAs transfer the mRNA information into proteins by delivering the corresponding amino acids into a ribosome to be linked together. Each 3 nitrogen bases forms a codon, and each codon corresponds to a specific tRNA that grabs a specific amino acid. The ribosome is made up of two subunits that snap together when it finds an mRNA. The amino acids that are linked together form long chains called proteins. These proteins, once completed, fold into 3-dimensional shapes that perform specific physical functions, folding with the help of enzymes. (Fig. 14: C & D).
Chicken and the Egg Issue? No, Protein and the DNA Issue
If you have paid attention thus far, you may have noticed what this biological conundrum may be. You see, the information to build proteins is stored in the DNA molecule, which must be transcribed into mRNA for the information to be transferred to proteins. Here's the catch: A protein itself, an enzyme called a replisome (DNA and RNA Polymerases), does the transcribing to make mRNA, which itself must be made from the information found in DNA. But a protein is needed for proteins to be made. So, which came first? The proteins, or the enzymes? This giant chicken and egg problem has led Abiogenesis research to propose that RNA was the first molecule. They posit that some "self-replicating" RNA molecules randomly formed that competed for molecular stability, replicating the best ones until you have the code for a simple cell.
We will see how the "RNA World" hypothesis utterly fails experimentally in a later article. But know for now that all RNA World research has failed to construct the correct handedness in the correct concentrations, or even create the correct forms of ribose, the backbone carbon sugar used in RNA. Origins of Life research is at a halt, and the researchers always invoke the hand of God multiple times throughout their experiments by something they call "unwarranted investigator input". This is where the chemist has to perform the chemistry to move the molecules in a life-friendly direction. Without the chemists, the chemistry of Abiogenesis simply wouldn't happen. As far as their yields and results, an intelligent agent played a role in the creation of life.



Comments